PrEP Prescriptions on the Rise: But More Work Remains
Topics
Pre-exposure prophylaxis (PrEP) is a highly effective HIV prevention strategy for people who want to take control of their health. It involves taking a pill every day. Studies show that daily PrEP can reduce the risk of acquisition of HIV via sex by more than 90% and reduce acquisition among people who inject drugs by more than 70%.
Given the strength of the scientific evidence and its ability to reduce new HIV infections, the National HIV/AIDS Strategy (NHAS) recognizes the importance of PrEP in ending HIV. The Strategy calls for full access to comprehensive PrEP services for those for whom it is appropriate and desired, along with support for medication adherence for those using PrEP.
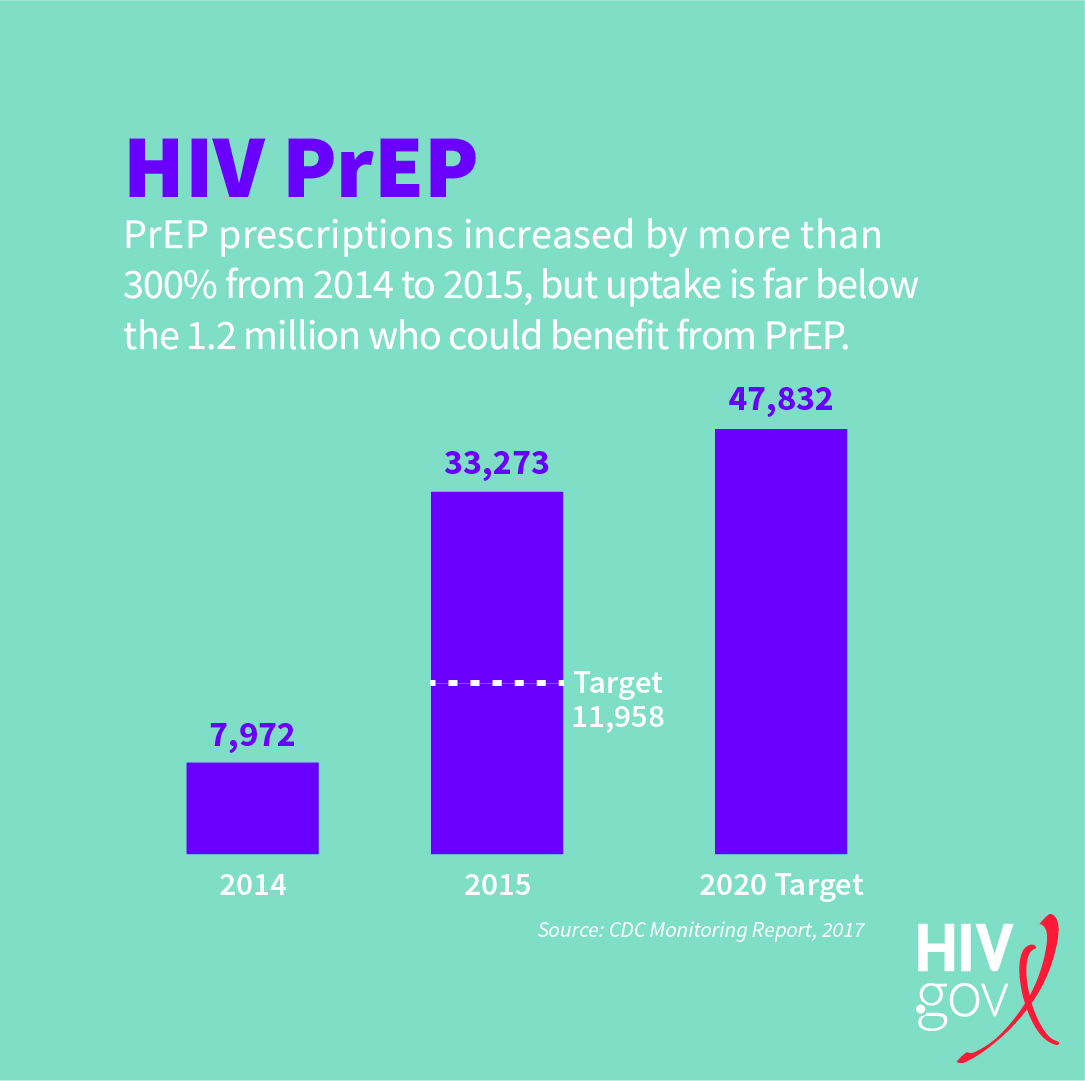
The Centers for Disease Control and Prevention (CDC) has estimated that there are more than 1.2 million women and men in the United States who can benefit from PrEP. In order to monitor the uptake of PrEP, the NHAS added a PrEP indicator as one of three developmental indicators introduced in 2016. The NHAS sets our target for increasing the number of adults prescribed PrEP by at least 500% between 2014 and 2020.
The Monitoring Report (PDF, 2.3 MB) released in July 2017 by the CDC shows that PrEP prescriptions increased more than 300% from 2014 to 2015. In 2015, 33,273 persons had been prescribed PrEP, which significantly exceeded our NHAS annual target for 2015 of 11,958. We are well on our way to achieving our 2020 target (47,832) ahead of schedule. This means that more aggressive targets may be needed in the future.
Analyses have not yet been conducted from the baseline for subgroups. However, an analysis published in the journal Clinical Infectious DiseasesExit Disclaimer found that among those prescribed PrEP in 2014*:
- 97% were men,
- Average age was 38 years,
- 98% lived in metropolitan areas, and
- 43% lived in the Western United States.
Further, an analysisExit Disclaimer (PDF, 260 KB) by Gilead Sciences, which manufactures the only currently FDA-approved form of PrEP, found the uptake of PrEP has been low among Blacks and Latinos from 2012-2015. (It should be noted that the Gilead analysis uses another data source and is measuring new PrEP prescriptions in a calendar year, whereas the data being used to monitor the NHAS indicator comes from the MarketScan database by Truven Health Analytics, which measures prevalence of PrEP prescriptions in a calendar year.) The Gilead analysis found that among all new PrEP prescriptions in 2015, only 10% were for Blacks/African-Americans and 12% for Latinos. The relatively low uptake of this effective HIV intervention among these populations is concerning given that they comprise the majority of annual HIV diagnoses: according to the CDC (PDF, 5.8 MB) in 2016 Blacks/African-Americans made up 44% of new HIV diagnoses and Latinos made up 25% of new HIV diagnoses. In comparison, over the same time-period 74% of new PrEP prescriptions were for Whites who made up 26% of new diagnoses in 2016.
These disparities highlight the need to continue to monitor uptake and expand upon efforts to increase the uptake of PrEP across the United States for those who can benefit most from PrEP and who desire to take control of their health. In December of 2016, the HIV PrEP Framework was released to serve as the federal government’s blueprint for scaling up PrEP. State and local jurisdictions and programs are encouraged to review the Framework, which describes seven essential components of a comprehensive PrEP response.
In order to scale up PrEP we must realize that multiple strategies will be essential in our response across the nation. This includes developing and implementing policies, guidance, and programs for PrEP screening, delivery, maintenance and adherence support, as well as mechanisms to pay for PrEP medication and related services. They also involve providing training and technical assistance to providers and grantees to expand capacity, build confidence in prescribing PrEP, and foster welcoming systems of care. Similarly they include educating those who may benefit from PrEP including addressing misconceptions and fears. Further, they include ongoing monitoring and evaluation to ensure programs are working effectively and efficiently and research to identify new drug mechanisms and to better understand how best to implement PrEP in different populations across different regions.
We must also realize that PrEP is a medical intervention, and many of those who may benefit most from PrEP are not insured or are often not comfortable or do not have a strong relationship with systems of care. Further, it is important to recognize that preventing HIV is but one component of an individual’s overall health and well-being. Often times it is necessary to address other competing needs such as care for other health issues, access to jobs, housing, and/or other services through navigators, coordinated systems of care, and collaborations with social services providers. Addressing these factors will be essential as we continue to expand access to PrEP and work to answer the Strategy’s call for full access to comprehensive PrEP services for all those for whom it is appropriate and desired.
In the next post in our continuing NHAS Indicators series, we will look at the indicator highlighting viral suppression disparities among people with diagnosed HIV. Stay tuned…
*The original analysis published in the journal Clinical Infectious Diseases using the MarketScan database by Truven Health Analytics, which serves as the data source for the PrEP indicator has introduced a new weighting method. This weighing method was used to produce new estimates for annual targets as well as for baseline, 2014, and 2015 data. The new estimates are included in the Monitoring Report published in July 2017.